

They are no longer associated directly with any particular atom or pair of atoms, but are free to wander throughout the whole sheet. These "spare" electrons in each carbon atom become delocalized over the whole of the sheet of atoms in one layer.
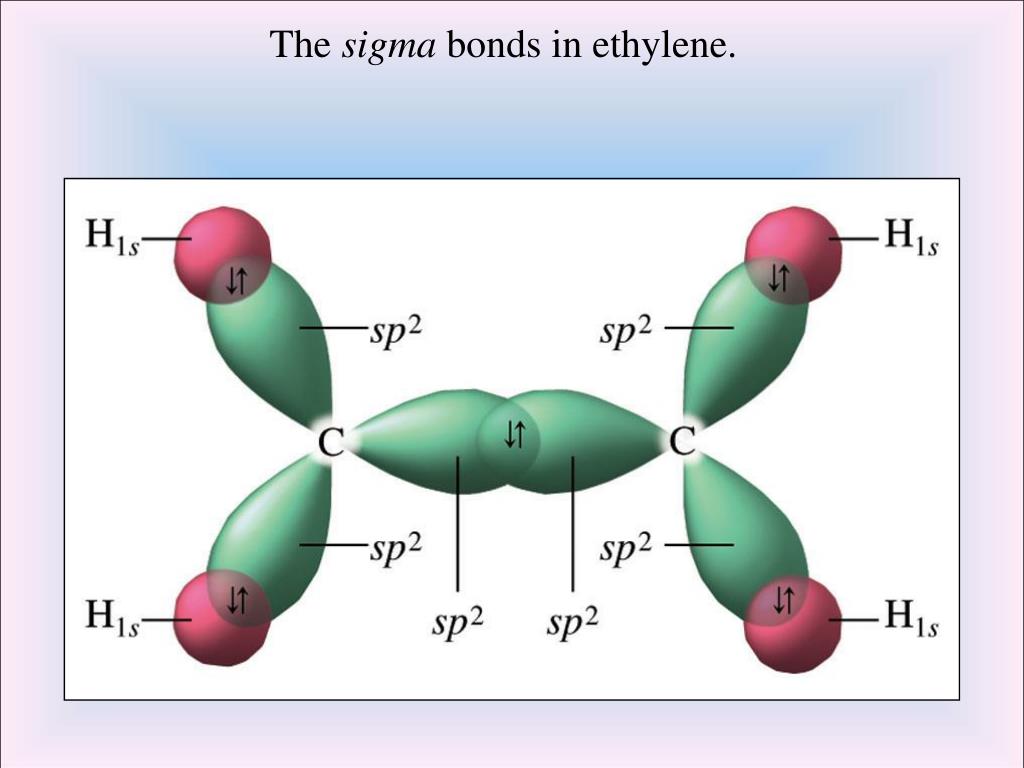
That leaves a fourth electron in the bonding level. You will need to use the BACK BUTTON on your browser to come back here afterwards.\)Įach carbon atom uses three of its electrons to form simple bonds to its three close neighbors. If this is the first set of questions you have done, please read the introductory page before you start. In truth, the carbon-hydrogen bond length is shorter than the carbon-carbon triple bond. To get the p orbitals to overlap and still see what is going on at the back of the molecule, you have to shorten the carbon-carbon distance completely out of proportion. Note: Forgive my artistic (in)ability! In particular, these diagrams are not to scale. Notice the different shades of red for the two different pi bonds. These pi bonds are at 90° to each other - one above and below the molecule, and the other in front of and behind the molecule. Sideways overlap between the two sets of p orbitals produces two pi bonds - each similar to the pi bond found in, say, ethene. The various p orbitals (now shown in slightly different reds to avoid confusion) are now close enough together that they overlap sideways. The sigma bonds are shown as orange in the next diagram. These are sigma bonds - just like those formed by end-to-end overlap of atomic orbitals in, say, ethane. The various atomic orbitals which are pointing towards each other now merge to give molecular orbitals, each containing a bonding pair of electrons. The two carbon atoms and two hydrogen atoms would look like this before they joined together: Don't confuse them with the shape of a p orbital. Notice that the two green lobes are two different hybrid orbitals - arranged as far apart from each other as possible.

What these look like in the atom (using the same colour coding) is: If your syllabus or teacher wants you to use just sp, then that's what you do. I prefer the older name, because it emphasises the fact that only one p orbital is involved - even if the 1 is technically unnecessary. Note The more modern name for what I call sp 1 hybrids is just sp hybrids. The new hybrid orbitals formed are called sp 1 hybrids, because they are made by an s orbital and a single p orbital reorganising themselves. They use the 2s electron and one of the 2p electrons, but leave the other 2p electrons unchanged. Important! If this isn't really clear to you, you must go and read the article about the bonding in methane.Įach carbon is only joining to two other atoms rather than four (as in methane or ethane) or three (as in ethene) and so when the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise two of the orbitals. This is exactly the same as happens whenever carbon forms bonds - whatever else it ends up joined to. The carbon atom doesn't have enough unpaired electrons to form four bonds (1 to the hydrogen and three to the other carbon), so it needs to promote one of the 2s 2 pair into the empty 2p z orbital. If you have read the ethene page, you will expect that ethyne is going to be more complicated than this simple structure suggests.Įthyne is built from hydrogen atoms (1s 1) and carbon atoms (1s 22s 22p x 12p y 1). In the diagram each line represents one pair of shared electrons. To understand ethene you also have to understand orbitals and the bonding in methane - sorry, there are no short-cuts! Depending on how many other pages you might have to refer to as well, return here later using the BACK button on your browser or the GO menu or HISTORY file - or via the Organic Bonding Menu (link from the bottom of each page in this section).Įthyne has a triple bond between the two carbon atoms. Unless you are already familiar with this, you should first read the page about ethene. Important! The approach on this page follows on from the similar (but very slightly easier) explanation of the bonding in ethene.

Bonding in ethyne (acetylene) - sp1 hybridisation


 0 kommentar(er)
0 kommentar(er)
